Organoboron compounds play a very important role in modern organic chemistry. As an extremely important synthon, these compounds allow a variety of conversions. As a kind of boron compound with great conversion potential, α-halogenated borate ester has important application value in organic synthesis and drug synthesis. Existing methods for synthesizing tri-substituted α-halogenated borate esters often require strong bases, extremely low temperatures, and organometallic reagents sensitive to air and moisture, which limits their application. Meanwhile, there is no good method for the synthesis of tetra-substituted α-halogenated borate esters as well as chiral α-halogenated borate esters.
Recently, the research team of Professor Xu Tao proposed a deoxygenative difunctionalization of carbonyls (DODC) strategy to realize the deoxygenative alkylboration of aldehydes (Angew. Chem., Int. Ed. 2022, e202214213) and deoxygenative arylboration of aldehydes (ACS Catal. 2022, 12, 14926). To solve the problem in the synthesis of α-halogenated borate esters, the research team realized the efficient synthesis of α-halogenated borate esters and chiral α-chlorinated borate esters by using aldehydes and ketones that are widely available or easily obtained as reaction precursors. The research outcome "Deoxygenative Haloboration and Enantioselective Chloroboration of Carbonyls" was published online in the Journal of American Chemical Society, an internationally renowned academic journal in the field of chemistry.
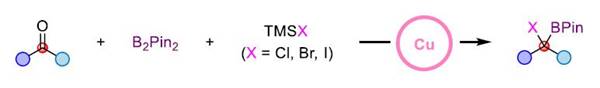
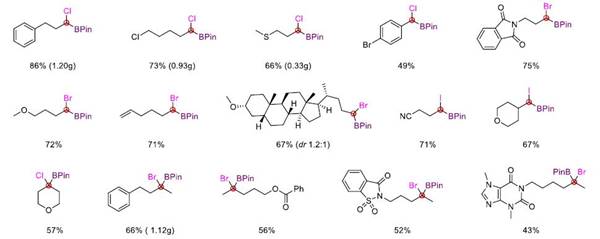
This reaction not only realizes the synthesis of tri-substituted α-halogenated borate esters but also can easily synthesize tetra-substituted α-halogenated borate esters that are difficult to obtain in traditional methods through this strategy. It has good compatibility with various functional groups such as ether, ester, ketone, amide, olefin, cyano, fluorine, chlorine, bromine, sulfur, and trifluoromethyl. In addition, the mild reaction conditions also make it possible to use this method for late-stage modification of complex molecules.
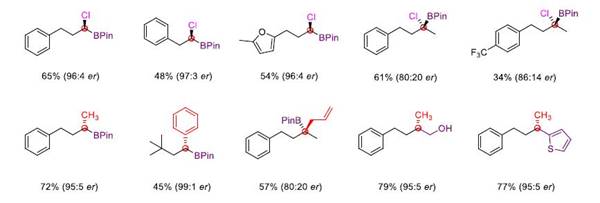
It is worth mentioning that the reaction can successfully realize the enantioselective chloroboration of carbonyl group through chiral catalysis, obtain chiral tri-substituted and tetra-substituted α-chlorinated borate ester, and it can easily realize multiple difunctionalization of aldehydes in only one or two steps, highlighting the great potential of this method in organic synthesis.
The research has developed a novel carbonyl deoxygenative haloboration reaction, which provides a very mild and simple method for the preparation of tri-substituted and tetra-substituted α-halogenated borate esters, and chiral chlorinated borate esters. More importantly, the diversified conversion of products opens up more opportunities for the rapid construction of complex molecules.
Wang Dong, a doctoral student of our school, is the first author of the paper, and Professor Xu Tao is the only corresponding author. The research work was funded by the National Natural Science Foundation of China.